EFFECT Trial
Michael Shen MD, Craig Asher MD, Mary Chandy MD, Tudor Scridon MD, Eric Dandes BS, Eduardo Vargas BS, Adrian Hernandez, MD, PhD, Howard Bush, MD, Kenneth Fromkin MD, Louis Ignarro PhD, Arthur Pilla PhD, Gian Novaro MD – Cleveland Clinic Florida, Weston, FL, Columbia University, New York, NY
A Randomized, Double-Blind, Parallel, Placebo-Controlled, Prospective Trial in 30 Patients with End-Stage Ischemic Heart Disease with Failed Medical Therapy and Revascularization Options (“No Option Patients“)
- First human clinical trial in 30 patients (15 active, 15 sham), self treated at home with PEMF, 2x daily, 30 minutes
- Active phase: 3 months, 2 month wash-out
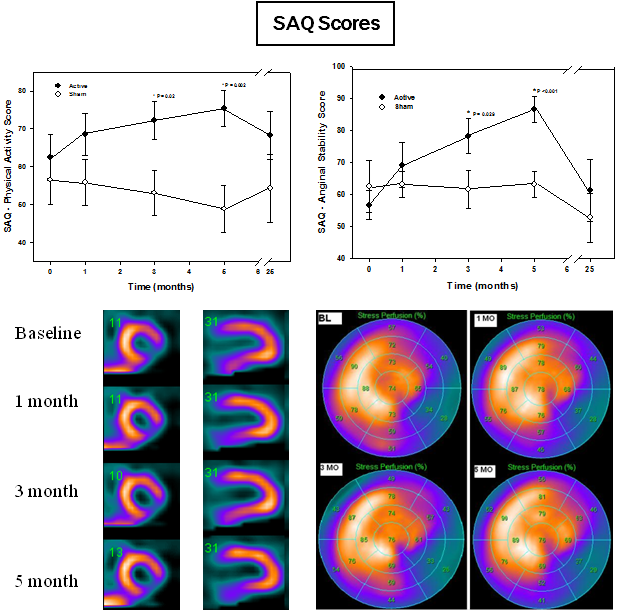
- Patients evaluated at 0, 1, 3 and 5 months: Seattle Angina Questionnaire (SAQ), SPECT imaging, and echo cardiogram
- Outcome measures: Improvement in angina and exercise tolerance; and Improvement in regional myocardial perfusion and function

[Electroceutical® for Ischemic Cardiomyopathy]
EFFECT Summary Results
- Significant between group differences – Improved SAQ subscales for: Anginal Frequency and Physical Activity in active cohort
- Significant within group improvements – Improved SAQ subscales for: Anginal Frequency, Severity and Physical Activity in active cohort; and Comparable to results seen in successful angioplasty patients
- Trend in improved perfusion in active cohort – requires more patients or longer therapy period for definitive results
- Echo: No significant differences between groups
- SPECT: No significant differences between groups – However, 3 patients in active cohort had 12-25% increase in perfusion compared to sham group