Who We Are
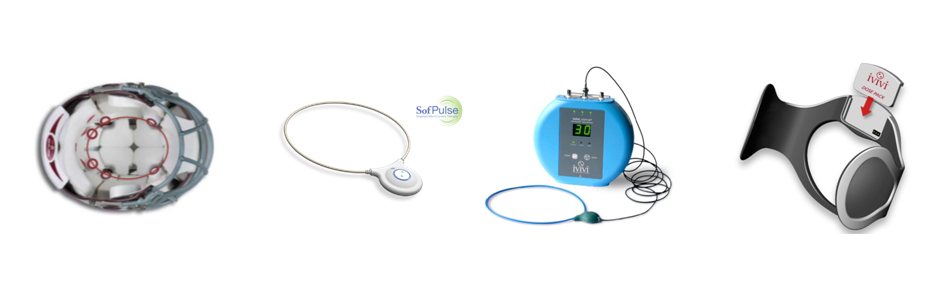
Endonovo Therapeutics (OTCQB: ENDV) is a commercial stage developer of non-invasive medical devices designed to deliver its proprietary Electroceutical® Therapy for the treatment of inflammatory conditions, cardiovascular diseases and central nervous system disorders.
Endonovo's Electroceutical® Therapy is FDA-Cleared for the palliative treatment of pain and post-surgical edema (swelling) and CE Marked for the promotion of wound healing and the palliative treatment of pain and post-surgical edema. Endonovo's Electroceutical® Therapy has CMS National Coverage for the treatment of chronic wounds. The Company's current pipeline of clinical-stage Electroceutical® Therapies targets cardiovascular diseases and central nervous system disorders, including ischemic heart disease, acute concussions, post-concussion syndrome, traumatic brain injury (TBI) and multiple sclerosis.
Our Vision
Endonovo means to renew from within. Our vision is to develop safe, wearable non-invasive medical devices to deliver our Electroceutical® Therapy, which harnesses bioelectricity to restore key electrochemical processes that initiate the anti-inflammatory and growth factor cascades necessary for healing to occur.